Detection of Canine Parvovirus by PCR and its association with some of risk factors
Detección de parvovirus canino por PCR y su asociación con algunos de los factores de riesgo
Detection of Canine Parvovirus by PCR and its association with some of risk factors
Revista MVZ Córdoba, vol. 23, no. 2, 2018
Universidad de Córdoba
Received: 09 January 2017
Accepted: 03 July 2017
Abstract: Objective. Canine parvovirus is one of the most important diseases of dogs all over the world that threaten their health. This study aimed to determine the prevalence of canine parvovirus in dogs referred to the Small Animal Clinic of Tehran University in Tehran province by genomic method of detection. Materials and methods. Diarrheal stool samples were obtained from 60 dogs during the years 2014-2016 and the presence of CPV was investigated by PCR method. Results. Prevalence of canine parvovirus infection in the studied dogs was 8.33% (5 0f 60). No significant differences between different age groups and breeds were found. However, infection was significantly higher in dogs with hemorrhagic diarrhea (3 of 25), but it was not statistically significant using chi-square test (p>0.05). Conclusions. This study showed that hemorrhagic diarrhea and lack of vaccination may be considered as important symptoms and risk factors for canine parvoviral infection in dogs and Dog owners in Iran should be aware of the fact that vaccination against CPV infection must be done carefully and on schedule to control and prevent the virus.
Keywords: Diarrhea, genomic detection, parvoviral infection, risk factor.
Resumen: Objetivo. El parvovirus canino es una de las enfermedades más importantes en perros en todo el mundo la cual amenaza su salud. El objetivo de este estudio fue determinar la prevalencia del parvovirus canino en perros remitidos a la Clínica de Pequeños Animales de la Universidad de Teherán en la provincia de Teherán por el método de detección del genoma. Materiales y Métodos. Se obtuvieron muestras de heces diarreicas de 60 perros durante los años 2014-2016 y se investigó la presencia de CPV por el método de PCR. Resultados. La prevalencia de infección por parvovirus canino en los perros estudiados fue del 8.33% (5 0f 60). No se encontraron diferencias significativas entre los diferentes grupos de edad y razas. Sin embargo, la infección fue significativamente mayor en perros con diarrea hemorrágica (3 de 25), pero no fue estadísticamente significativa usando la prueba de chi-cuadrada (p>0.05). Conclusiones. Este estudio demostró que la diarrea hemorrágica y la falta de vacunación pueden considerarse como síntomas y factores de riesgo importantes de la infección por parvovirus canino en perros, y que los propietarios de perros en Irán deben ser conscientes del hecho de que la vacunación contra la infección CPV debe hacerse con cuidado y a tiempo para controlar y prevenir el virus.
Palabras clave: Diarrea, detección genómica, infección por parvovirus, factor de riesgo.
INTRODUCTION
Nowadays, in some people’s life dog has become indispensable as a companion. Dogs have diversified utility ranging from tracking, hunting, instruments of war, bomb detecting squad and healer of both physical and emotional problems of humans, detecting criminals and guiding blind people (1). In view of the need of dogs it is inevitable to study the various diseases that are important in dog’s life. Among the viral diseases the predominantly occurring diseases includes canine parvoviral infection, canine distemper, corona virus infection, canine hepatitis, canine parainfluenza and rabies (2).
The family Parvoviridae comprises two subfamilies, Parvovirinae and Densovirinae, infecting vertebrates and insects, respectively. Based on ICTV 10th report, eight genera are included in the subfamily Parvovirinae, namely Amdoparvovirus, Aveparvovirus, Bocaparvovirus, Copiparvovirus, Dependoparvovirus, Erythroparvovirus Protoparvovirus and Tetraparvovirus. Canine parvovirus (CPV) belongs to genus Protoparvovirus. Canine parvovirus (CPV) was first identified in 1978 (3). It has a single-stranded DNA genome length of about 5.200 nucleotides. Parvoviruses are small (diameter of 25 nm), non-enveloped that their virion consists of a spherical capsid, which is composed by three proteins and contains a linear, single- strand DNA molecule (4). The virus is a major pathogen of dogs and may cause myocarditis in young puppies. It causes hemorrhagic gastroenteritis in older animals. Enteric form of disease has predominated and it persists as a major problem in breeding kennels, or where vaccination is widely practiced (5). Parvovirus infection is most commonly manifested with signs like, vomiting, bloody diarrhea and severe leukopenia (6). In fact virus causes a highly contagious disease that can rapidly spread through a population of dogs. Virus is generally shed extensively for 7–12 days, but long-term excretion may occur as well (7).
Although there are vaccination programs against disease, vaccine failure are common. In organized kennel, an outbreak may result in very high morbidity and mortality rates. Therefore early and fast diagnosis is necessary so that infected dogs can be isolated and supportive treatment can be administered for disease (3).
Clinical diagnosis of CPV infection is difficult because the main clinical signs of disease (vomiting and diarrhea) are common to other enteric diseases. DNA based detection using PCR is one of the most precise and rapid method for detection of CPV. This assay requires only 2 to 4 hours for detection of viral nucleic acid (8). The prevalence study may some way beneficial to check the infection among the susceptible population. Furthermore, the epidemiology regarding CPV in the study area is very scarce. Outbreaks of CPV have been reported from many countries worldwide (9) and there are some reports about the presence of virus based on clinical diagnosis or detection of CPV from fecal samples in Iran (10,11,12). However, as we researched, there is no report of CPV from blood samples in Iran. Therefore, the present study was conducted to determine the prevalence of CPV infection among dogs in the Tehran city of Iran, by PCR, for which effective control measures could be carried out.
MATERIALS AND METHODS
Blood sampling and DNA extraction. This study was a cross-sectional survey and the target group was all dogs who referred to Small Animal Clinic of Tehran University and have shown general gastroenteritis symptoms. The fecal samples were obtained during the period of 2014–2016 and were analyzed to detect CPV NS1 gene. Dogs were divided into two age groups (less or more than 6 months), 4 different breeds (terrier, German shepherd, Doberman Pinschers and mixed) and 2 groups based on clinical symptoms (hemorrhagic diarrhea and non-hemorrhagic diarrhea), using Fisher’s exact test. The samples were stored at -20 until testing. Then, DNA was extracted using tissue and blood DNA extraction kit (DYNABIO, Cat No: KI0015) according to the manufacturer’s instructions.
Detection of CPV by PCR. The presence of CPV was detected using the primers designed by Beacon designer software. The sequences of the Forward and reverse primers were 5’- GAATCAACCATGGCTAAC -3’ and 5’- GTCTTGACTCTGAGGATTG -3’ respectively. Each PCR reaction was performed in a final volume of 25 µl containing 11 µl of deionized sterile water, 10 µl of Taq DNA Polymerase 2x Mix Red-Mgcl2 2 mM (GeneAll, Cat. No. A180301), 1 pmol of each primer and 2 µl of DNA template.
The thermal cycling conditions for the amplification were 1 cycle for 4 min at 94°C, 35 cycles of 30 s at 94°C, 45 s at 59°C and 45 s at 72°C, with a final extension step of 5 min at 72°C. Positive and negative controls (from Veterinary Laboratories Agency, UK) were included in each analysis. 6 µl of the amplified products were loaded on a 1.3% agarose gel, and visualized by staining with ethidium bromide and compared to DNA markers (100 base pair ladder, Fermentas).
Sequencing. Two PCR positive samples in a volume of 50 ml were sent to Bioneer Company and sequencing performed on the ABI 3730XL DNA Analyzer by the Sanger method.
RESULTS
Infection and statistics. 60 fecal samples tested by PCR. Of these, 5 samples (8.33%) were positive for CPV NS1 nucleotide fragment. 3 (of 25) positive cases (14.28%) had hemorrhagic diarrhea (Table 1). The distribution of the disease in two age groups of the tested dogs (less or more than 6 months) is mentioned in Table 2 and the distribution of positive cases in 4 different breeds (terrier, German shepherd, Doberman Pinschers and mixed) is listed in Table 3. No significant differences between different age groups and breeds were found. However, infection was significantly higher in dogs with hemorrhagic diarrhea (3 of 25), but it was not statistically significant (p>0.05) using chi-square test (IBM SPSS.22).
| Total | Positive (Percent) | Positive (Number) | symptom |
| 35 | 5.71 | 2 | Non-hemorrhagic diarrhea |
| 25 | 12 | 3 | Hemorrhagic diarrhea |
| 60 | 8.33 | 5 | Total |
| Total | Positive (Percent) | Positive (Number) | Age group |
| 37 | 8.11 | 3 | Less than 6 months |
| 23 | 8.7 | 2 | More than 6 months |
| 60 | 8.33 | 5 | Total |
| Total | Positive (Percent) | Positive (Number) | Breed |
| 11 | 9.09 | 1 | terrier |
| 8 | 0 | 0 | German shepherd |
| 11 | 9.09 | 1 | Doberman Pinschers |
| 30 | 10.00 | 3 | mixed |
| 60 | 8.33 | 5 | Total |
PCR. CPV was detected using PCR test specific for the CPV-NS1 nucleotide fragment. The CPV-specific band with the size of 150 bp was detected in DNA positive control. The positive PCR products were in the same size as those from the positive control (Figure 1).
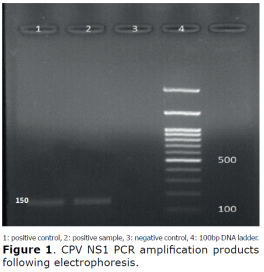
Sequencing. After sequencing, the BLAST of the read sequences, confirmed the presence of CPV-NS1 fragment in the positive samples (Figure 2).

There is 97% identity between PCR product sequence and canine parvovirus NS1 gene.
DISCUSSION
Canine parvovirus (CPV) is the most important enteric virus responsible for severe enteritis infecting canids worldwide. It has been most frequently detected in both diarrheal and normal feces. It has been approved CPV-2 specifically associate with diarrheal cases (13). Despite its DNA genome, the virus possesses a high genetic substitution rate which is responsible for uninterrupted antigenic evolution and rapid appearance of new antigenic variants (6).
There are several methods used for detection of this virus: electron microscopy (EM), virus isolation (VI), latex agglutination (LA), hemagglutination (HA), in situ hybridization (ISH), ELISA, and polymerase chain reaction (PCR) analysis (5,7,8,9).
Although EM and VI are highly specific and sensitive, they often are too time consuming and expensive for routine use in a clinical setting. The LA is a rapid test, but it lacks specificity. The HA lacks reliability without a confirmatory inhibition test, and has the additional disadvantage of requiring a continuous supply of fresh erythrocytes (14). The ELISA may be less sensitive than EM (15).
Polymerase chain reaction analysis has been widely applied for laboratory diagnosis because of its sensitivity and specificity (2,14,17). In fact, PCR becomes an important part of modern diagnostic methods. Currently, the standard method for diagnosing the presence of viral pathogens in clinical samples relies on culture and other techniques which are time consuming and cumbersome. However, active research is under way using new molecular methods such as PCR (9). PCR is shown in previous studies that may detect virus even at a concentration as low as about 10 infectious particles per reaction, corresponding to a virus titer of about 103 PFU/ml of unprocessed fecal material (8). This sensitivity is good agreement with results of a published study by another group using different primers, pretreatment protocols and PCR conditions (13). For these mentioned reasons PCR was used for detecting CPV.
The purpose of this study was to detect CPV in dogs with diarrhea distributed throughout Tehran province of Iran between 2014 and 2016 and to identify some risk factors associated with CPV infection. From 60 dogs suspected of being infected with CPV, historical data and clinical signs were collected. Fecal samples were screened for CPV by PCR assay and those positive were confirmed by sequencing. The study revealed an overall prevalence of canine parvovirus infection in suspected dogs to be 8.33%. Previously, Mochizuki et al (14) detected CPV-2 DNA from fecal samples of dogs with diarrhea by nested PCR. They found CPV-2 DNA in 22 out of 59 (37.3%) fecal samples (14). Schunck et al (8) detected CPV-2 DNA from fecal specimens derived from enteritic dogs by single round PCR. Using this method, they established the presence of CPV-2 DNA in 54 out of 65 (83.1%) samples tested. Also, the presence of CPV in India has been confirmed by Ramadass and Khader. Strain of CPV present in India has been documented to be CPV 2a (4).
Previous studies reported that dogs in Thailand were infected with CPV-2 by neutralizing antibody tests. CPV is progressively spreading in Italy, Portugal, Spain and Germany and has been detected in Vietnam also (15,16,17,18).
In the present study, a low incidence rate was observed (8.33%). The above finding wasn’t in accordance with those of Phukan et al who reported 42.29% incidence of CPV infection among suspected dogs by sandwich ELISA method in Assam, India (19). Srinivas et al also reported 53.90% incidence by PCR assay using H primer in five states of southern India (20). However, higher incidences were reported by Phukan et al such as 64% positive by sandwich ELISA and 76% by indirect ELISA (21). Singh et al. reported a higher incidence of 63%. Such high incidence might be due to prevalence of endemic infection in the population under study (22) or the type of diagnostic test that each group used. Furthermore, the pattern of CPV induced disease in a population is largely influenced by the susceptibility of host, environmental conditions such as housing, hygiene, population density, and pathogenicity of the infectious agent (23).
Joshi et al (24) reported lower incidences up to 23.02% by counter immunoelectrophoresis. However, these variations observed in the prevalence are difficult to explain due to the different study area and difference in the methods of sample analysis.
In this study breed-wise distribution of CPV revealed mixed breeds (10.0%) were more prone to CPV infection than other breeds. However, this higher CPV prevalence wasn’t statistically significant (p>0.05) using chi-square test (IBM SPSS 22). Similar findings were reported previously (25) where 27.23% incidence was observed in the local breed. Archana et al (26) also reported higher prevalence of 56.9% in nondescript dogs. More incidences in mixed breeds might be due higher population of this breed making their close proximity to spread the infection and poorness or absence of vaccination schedule being followed by the owners of mixed breed presumably due to lack of awareness among them. No specific comments can be made on breed susceptibility as the population density of breeds varies from one geographical area to another (26).
Age-wise prevalence was found to be more between the age group of below 6 months. However, this higher CPV prevalence wasn’t statistically significant (p>0.05) using chi-square test (IBM SPSS 22). This higher prevalence rate below 6 months also reported earlier (20,27,28,29). The higher incidence of CPV below 6 months might be due to the affinity of the virus for rapidly multiplying intestinal crypt cells in weaning pups with higher mitotic index due to changes in bacterial flora as well as in the diet due to weaning (30,31). The fall in maternal antibody level after 3 months age might be one of the predisposing factors, which make the age group of 3-6 months old more prone to CPV and as they advance in age become prone to the infection in endemic areas due to decline in protective titers (25,31). In contrast Phukan et al. reported highest incidence in the age group of 7-12 months followed by 1-6 months, 13 months and above (19).The prevalence in the age more than 6 months might be due to improper age or timing of vaccination, non-boostering of the animals and improper maintenance of cold chain for storage of vaccines (31). We found that dogs with hemorrhagic diarrhea are mostly infected with parvovirus. However, this higher CPV prevalence wasn’t statistically significant (p>0.05) using chi-square test (IBM SPSS 22). Since virus replication requires rapidly dividing cells of fetuses and newborns or of hematopoietic and intestinal tissues of young and adult animals its presence in hemorrhagic diarrhea is predictable (3).
In this study we found the overall prevalence of CPV in dogs was 8.33% using PCR, and due to the fact that all parvoviruses are highly stable in the environment, as they are extremely resistant to pH and temperature changes and to treatment, vaccination against CPV infection must be done carefully and on schedule to control and prevent the CPV disease.
Acknowledgements
This work was carried out within the framework of a project sponsored by Shahrekord University and we gratefully acknowledge the help by Mrs. Fatemeh Kabiri.
REFRENCES
1. Bargujar J, Ahuja A, Bihani DK, Kataria N, Dhuria D. Studies on prevalence, clinical manifestations and therapeutic management in dogs suffering from canine parvovirus infection. JCDR. 2011; 7:9-16.
2. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. USA: Elsevier Academic Press; 2005.
3. Muzyczka N, Berns KI. Parvoviridae: The Viruses and Their Replication. Fields Virology. 3th edition. Knipe DM, Howley PM, Lippincott Williams & Wilkins: Philadephia, USA; 2001.
4. Sagazio P, Tempesta M, Buonavoglia D, Cirone F, Bounavoglia C. Antigenic characterization of canine parvovirus strains isolated in Italy. J Virol Methods. 1998; 73:197-200.
5. Decaro N, Desario C, Campolo M, Elia G, Martella V, Ricci D, Lorusso E, Buonavoglia C. Clinical and virological findings in pups naturally infected by canine parvovirus type 2 Glu-426 mutant. J Vet Diagn Invest. 2005; 17:133–138.
6. Shackelton LA, Parrish CR, Truyen U, Holmes EC. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc Natl Acad Sci USA. 2005; 102:379–384.
7. Cho HS, Song JE, Park YS. Diagnosis of the canine parvovirus in fecal samples by in situ hybridization. Indian Vet J. 2004; 81:855–859.
8. Schunck B, Kraft W, Truyen U. A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J Virol Methods. 1995; 55:427–433.
9. Sharma P, Rastogi A, Kukreti K, Singh Narwal P. Sensitivity assay of polymerase chain reaction for detection of Canine Parvo Virus infection in dogs. OJCD. 2012; 2:45-47.
10. Askari FiroozjaiiH, Jafari Shoorijeh S, Mohammadi A, Tamadon A. Characterization of Iranian isolates of canine parvovirus in fecal samples using polymerase chain reaction assay. Iran J Biotechnol. 2011; 9(1):63-68.
11. Dastmalchi Saei H, Javadi S, Akbari S, Hadian N, Zarza E. Molecular characterization of canine parvovirus (CPV) antigenic variants from healthy and diarrheic dogs in Urmia region, Iran. Iran J Vet Med. 2017; 11(1):9-19.
12. Mosallanejad B, Ghorbanpoor Najafabadi M, Avizeh R. The first report of concurrent detection of canine parvovirus and coronavirus in diarrhoeic dogs of Iran. Iran J Vet Res. 2008; 9(3):284-286.
13. Uwatoko K, Sunairi M, Nakajima M, Yamaura K. Rapid method utilizing the polymerase chain reaction for detection of canine parvovirus in feces of diarrhei dogs. Vet Microbiol. 1995; 43:315–323.
14. Mochizuki M, San Gabriel MC, Nakatani H, Yoshida M. Comparison of polymerase chain reaction with virus isolation and hemagglutination assays for the detection of canine parvoviruses in fecal specimens. Res Vet Sci. 1993; 55:60–63.
15. Decaro N, Elia G, Martella V, Campolo M, Desario C, Camero M, et al. Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology. J Virol Methods. 2006; 133:92–99.
16. Decaro N, Martella V, Desario C, Bellacicco AL, Camero M, Manna L, et al. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain. J Vet Med B Infect Dis Vet Public Health. 2006; 53:468–72.
17. Martella V, Cavalli A, Pratelli A, Bozzo G, Camero M, Buonavoglia D, et al. A canine parvovirus mutant is spreading in Italy. J Clin Microbiol. 2004; 42:1333–1336.
18. Nakamura M, Tohya Y, Miyazawa T, Mochizuki M, Phung HT, Nguyen NH, et al. A novel antigenic variant of canine parvovirus from a Vietnamese dog. Arch Virol. 2004; 149:2261–2269.
19. Phukan A, Baishya B, Deka D, Boro PK. Prevalence of canine parvovirus infection in Assam. Indian Vet J. 2010; 87:972-974.
20. Srinivas VMV, Mukhopadhyay HK, Antony PX, Pillai RM. Molecular epidemiology of canine parvovirus in Southern India. Vet World. 2013; 6(10):744-749.
21. Phukan A, Sarma DK, Deka D, Boro P. Standardization of ELISA for detection of canine parvovirus infection. Indian Vet J. 2005; 82:355-356.
22. Singh D, Verma AK, Kumar A, Srivastava M, Singh SK, Tripathi AK, et al. Detection of canine parvo virus by polymerase chain reaction assay and its prevalence in dogs in and around Mathura, Uttar Pradesh, India. Am J Biochem Mol Biol. 2013; 3(2):264-270.
23. Nandi S, Kumar M, Chidri S, Chauhan RS. Current status of canine parvo virus infection in dogs in India and its pathogenesis. Indian J Vet Pathol. 2008; 32(2):150-157.
24. Joshi DV, Singh SP, Rao VDP, Patel BJ. Diagnosis of canine parvovirus infection by counter immunoelectrophoresis. Indian Vet J. 2000; 77:899-900.
25. Tajpara MM, Jhala MK, Rank DN, Joshi CG. Incidence of canine parvovirus in diarrhoeic dogs by polymerase chain reaction. Indian Vet J. 2009; 86:238-241.
26. Archana Shukla PC, Gupta DK, Kumar B. Epidemiology on canine parvovirus infection. Indian J Vet Sci. 2009; 18(2):42-44.
27. Parthiban S, Mukhopadhyay HK, Panneer D, Antony PX, Pillai RM. Isolation and typing of canine parvovirus in CRFK cell line in Puducherry, South India. Indian J Microbiol. 2011; 51(4):456-460.
28. Xu J, Guo HC, Wei YQ, Shu L, Wang J, Li JS, Cao SZ, Sun SQ. Phylogenetic analysis of canine parvovirus isolates from Sichuan and Gansu Provinces of China in 2011. Transbound Emerg Dis. 2013; 62(1):91-95.
29. Mohanraj J, Mukhopadhyay HK, Thanislass J, Antony PX, Pillai RM. Isolation, molecular characterization and phylogenetic analysis of canine parvovirus. Infect Genet Evol 2010; 10:1237-1241.
30. Deka D, Phukan A, Sarma DK. Epidemiology of parvovirus and coronavirus infections in dogs in Assam. Indian Vet . 2013; 90(9):49-51.
31. Stepita ME, Bain MJ, Kass PH. Frequency of CPV infection in vaccinated puppies that attended puppy socialization classes. J Am Anim Hosp Assoc. 2013; 49:95-100.